Maleimide Surfaces
for oriented covalent coupling (immobilization) of thiolated biochemical species
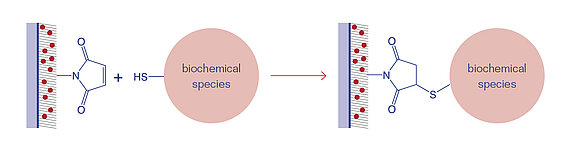
Maleimide-esters react immediately with Thiol-groups of biochemical species. The Thiol-groups can be either natively present in the (bio)molecule, e.g. through the amino acid cysteine in proteins, produced via reductive cleavage of disulfide bonds with a reducing agent such as Dithiothreitol (DTT, Cleland´s Reagent), or selectively introduced e.g. with 2-Iminothiolane (Traut's reagent) for amine-containing molecules.
Similar to NHS-esters, Maleimide surfaces are susceptible to hydrolysis, and thus, should be processed promptly after opening the sealed bags.
PolyAn equips glass slides, coverslips, polymer slides and 96-well plates with reactive surfaces. Please do not hesitate to contact us, if you would like to functionalize a different format or substrate with our 3D-Maleimide surface.
Selected Publications
Maleimide surface for the immobilization of Lipopolysaccharides:
- Chen, D. et al., `A Bioactive Synthetic Outer-Core Oligosaccharide Derived from a Klebsiella pneumonia Lipopolysaccharide for Bacteria Recognition´, Chem. Eur. J., 2023, 29, 202203408. DOI: 10.1002/chem.202203408.
Maleimide surface for the immobilization of Peptides:
- Randriantsilefisoa, R. et al., `Highly sensitive detection of antibodies in a soft bioactive three-dimensional bioorthogonal hydrogel´, J. Mater. Chem. B, 2019, 7, 3220. DOI: 10.1039/c9tb00234k.
- Palermo, A. et al., `Identification of a Tetanus Toxin Specific Epitope in Single Amino Acid Resolution´, Biotechnol. J., 2017, 12, 1700197. DOI: 10.1002/biot.201700197.
Maleimide surface for the immobilization of Glycans:
- Broecker, F. et al., `Synthetic Glycan Microarrays´, Meth. Mol. Biol., 2017, 1518, 227. DOI: 10.1007/978-1-4939-6584-7_15.
- Götze, S. et al., `Investigation of the protective properties of glycosylphosphatidylinositol-based vaccine candidates in a Toxoplasma gondii mouse challenge model´, Glycobiology, 2015, 25, 984. DOI: 10.1093/glycob/cwv040.
- Malik, A. et al., `Immunological Evaluation of Synthetic Glycosylphosphatidylinositol Glycoconjugates as Vaccine Candidates against Malaria´, Chem. Biol., 2020, 15, 171. DOI: 10.1021/acschembio.9b00739.
