PolyAn Red4 Multiplex Beads
PolyAn offers a set of 30 bead populations (30-plex) that can be distinguished both by different fluorescence intensities of our PolyAn Red4 dye in the APC channel (Excitation 590–680 nm, Emission 660–780 nm) and three different bead sizes (3.5 / 5.5 / 8.5 μm).
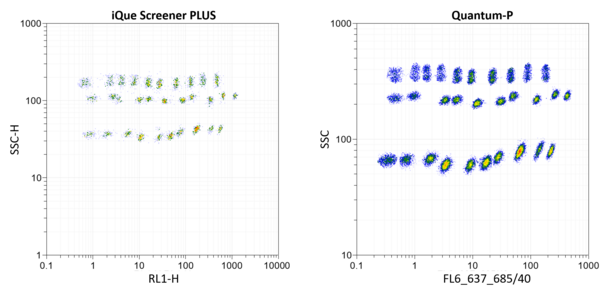
PolyAn Red4 Multiplex Beads provide a platform for the design of multiplex assays that can be run on a wide range of standard flow cytometers. The image below illustrates the read-out of all 30 Red4 bead populations using different flow cytometers (left: IntelliCyt® iQue Screener PLUS from Sartorius, right: Quantum-P from Quantum Analysis).
Products
The standard packaging volume is 1.5 mL/population with a solids content of 0.5%. A custom modification with antibodies, peptides or oligonucleotides is available upon request.
Principle of Bead-based Immunoassays
The principle of bead-based sandwich immunoassays for the detections of antigens or antibodies using PolyAn Red4 Multiplex Beads and flow cytometry is illustrated below.
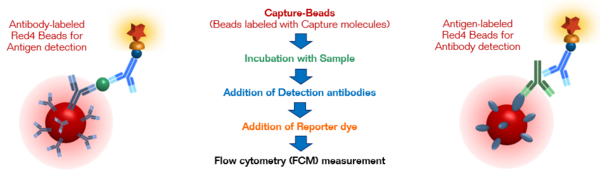
Each Red4 bead population is encoded with a specific fluorescence intensity, enabeling the classification of the bead populations by their different emission intensities. As each Red4 bead population is labeled with specific capture molecules (e.g. antibodies or antigens), the respective analytes specifically bind to their corresponding "Capture-Beads" and can then be quantified via the emission intensity of a reporter dye.
A flyer showing the use of PolyAn Red4 Multiplex Beads for the multiplexed detection in immunoassays using flow cytometry can be downloaded here.
Selected Publications
- Held, S. et al., `Physiological shedding and C-terminal proteolytic processing of TMEM106B´, Cell Reports, 2025, 44, 115107. DOI: 10.1016/j.celrep.2024.115107.
- Joseph, E. et al., `Design, Synthesis and Preclinical Evaluation of a brain-permeable PET Tracer for P2Y12R Imaging in the Brain´, ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-5461s.
- Laposchan, S. et al., `Development of Motif-Specific Monoclonal Antibodies for Global Protein Citrullination Detection with Minimal Cross-Reactivity to Homocitrullination´, bioRxiv, 2025, DOI: 10.1101/2025.03.27.645732.
- Pfisterer, M. et al., `The Aurora B-controlled PP1/RepoMan complex determines the spatial and temporal distribution of mitotic H2B S6 phosphorylation´, Open Biology, 2024, 14, 230460. DOI: 10.1098/rsob.230460.
- Papadogianni, G. et al., `Establishment and Functional Characterization of Murine Monoclonal Antibodies Recognizing Neuritin´, Antibodies, 2023, 12, 28. DOI: 10.3390/antib12020028.
- Riemenschneider, H. et al., `Targeting the glycine‑rich domain of TDP‑43 with antibodies prevents its aggregation in vitro and reduces neurofilament levels in vivo´, Acta Neuropath. Commun., 2023, 11, 112. DOI: 10.1186/s40478-023-01592-z.
- Rudan Njavro, J.et al., `Beneficial Effect of ACI-24 Vaccination on A-beta Plaque Pathology and Microglial Phenotypes in an Amyloidosis Mouse Model´, Cells, 2023, 12, 79. DOI: 10.3390/cells12010079.
- Kutzner, K. et al., `Phosphorylation of serine-893 in CARD11 suppresses the formation and activity of the CARD11-BCL10-MALT1 complex in T and B cells´, Sci. Signal., 2022, 15, 3083. DOI: 10.1126/scisignal.abk3083.
- Mishima, E. et al., `A non-canonical vitamin K cycle is a potent ferroptosis suppressor´, Nature, 2022, 608, 778. DOI: 10.1038/s41586-022-05022-3.
- Xin, S. et al., `MS4A15 drives ferroptosis resistance through calcium-restricted lipid remodeling´, Cell Death Differ., 2022, 29, 670. DOI: 10.1038/s41418-021-00883-z.
- Egia-Mendikute, L. et al., `Sensitive detection of SARS-CoV-2 seroconversion by flow cytometry reveals the presence of nucleoprotein-reactive antibodies in unexposed individuals´, Commun. Biol., 2021, 4, 485. DOI: 10.1038/s42003-021-02011-6.
