PolyAn Beads for Multiplex Assays
PolyAn’s Multiplex Beads are designed for the development of multiplex assays that can be analyzed using a wide range of flow cytometers or fluorescence imaging-based platforms (e.g. fluorescence microscope systems):
- Flow Cytometry Systems: PolyAn Red4 Multiplex Beads - up to 30 populations of single color encoded beads
- Flow Cytometry Systems: PolyAn Blue Multiplex Beads - up to 10 populations of single color encoded beads, that can be combined with the 30-plex PolyAn Red4 Multiplex Bead Set
- Fluorescence microscope systems: Dual color-encoded Multiplex Beads - up to 18 populations of dual color encoded beads
- Customized development of multiplex sets for OEM-applications
PolyAn’s multiplex assay technology utilizes multiple bead populations differentiated by size and different levels of fluorescence intensity and/or fluorophores. With multiple sizes of beads and multiple levels of fluorescence intensity in each bead size, the PolyAn Multiplex technology can measure up to 30 analytes simultaneously in a single reaction.
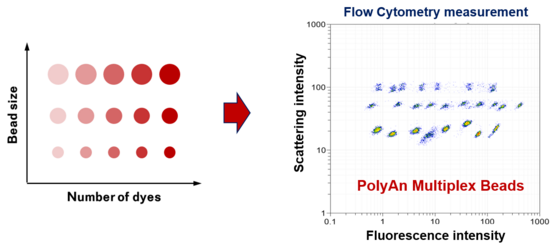
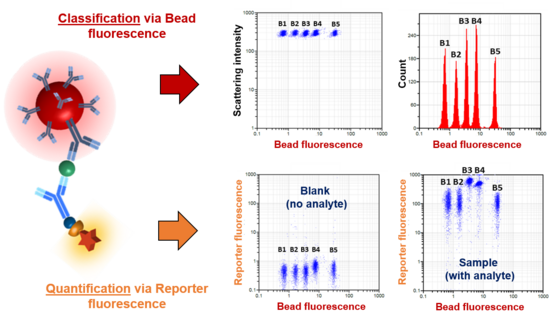
Each bead population can be conjugated with a specific biomolecule (such as an oligonucleotide, peptide or antibody) on the surface and serves as the capture bead for that particular analyte. When a selected panel of capture beads are mixed together and incubated with an unknown sample containing the target analytes, each analyte will be bound by its specific capture bead.
After washing, detection antibodies are added and each detection antibody will bind to its specific analyte bound on the capture beads, thus forming capture bead-analyte-detection antibody sandwiches.
PolyAn offers the design of multiplex beads for proprietary analytics systems. Please do not hesitate to contact us, if you are interested in the OEM-production of multiplex beads for your specific systems.
Selected Publications
- Held, S. et al., `Physiological shedding and C-terminal proteolytic processing of TMEM106B´, Cell Reports, 2025, 44, 115107. DOI: 10.1016/j.celrep.2024.115107.
- Joseph, E. et al., `Design, Synthesis and Preclinical Evaluation of a brain-permeable PET Tracer for P2Y12R Imaging in the Brain´, ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-5461s.
- Laposchan, S. et al., `Development of Motif-Specific Monoclonal Antibodies for Global Protein Citrullination Detection with Minimal Cross-Reactivity to Homocitrullination´, bioRxiv, 2025, DOI: 10.1101/2025.03.27.645732.
- Pfisterer, M. et al., `The Aurora B-controlled PP1/RepoMan complex determines the spatial and temporal distribution of mitotic H2B S6 phosphorylation´, Open Biology, 2024, 14, 230460. DOI: 10.1098/rsob.230460.
- Papadogianni, G. et al., `Establishment and Functional Characterization of Murine Monoclonal Antibodies […]´, Antibodies, 2023, 12, 28. DOI: 10.3390/antib12020028.
- Riemenschneider, H. et al., `Targeting the glycine‑rich domain of TDP‑43 with antibodies prevents its aggregation in vitro and reduces neurofilament levels in vivo´, Acta Neuropath. Commun., 2023, 11, 112. DOI: 10.1186/s40478-023-01592-z.
- Rudan Njavro, J.et al., `Beneficial Effect of ACI-24 Vaccination on A-beta Plaque Pathology and Microglial Phenotypes in an Amyloidosis Mouse Model´, Cells, 2023, 12, 79. DOI: 10.3390/cells12010079.
- Kutzner, K. et al., `Phosphorylation of serine-893 in CARD11 suppresses the formation and activity of the CARD11-BCL10-MALT1 complex in T and B cells´, Sci. Signal., 2022, 15, 3083. DOI: 10.1126/scisignal.abk3083.
- Mishima, E. et al., `A non-canonical vitamin K cycle is a potent ferroptosis suppressor´, Nature, 2022, 608, 778. DOI: 10.1038/s41586-022-05022-3.
- Xin, S. et al., `MS4A15 drives ferroptosis resistance through calcium-restricted lipid remodeling´, Cell Death Differ., 2022, 29, 670. DOI: 10.1038/s41418-021-00883-z.
- Egia-Mendikute, L. et al., `Sensitive detection of SARS-CoV-2 seroconversion by flow cytometry reveals the presence of nucleoprotein-reactive antibodies in unexposed individuals´, Commun. Biol., 2021, 4, 485. DOI: 10.1038/s42003-021-02011-6.
