2D-Azide Surface
for reaction with Alkyne groups and DBCO-modified molecules via Click Chemistry
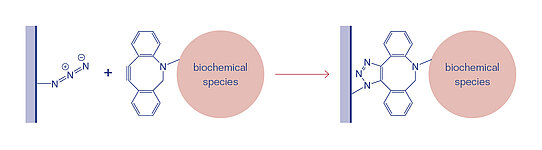
Azide groups are reactive funtional groups for Click chemistry reactions. Click chemistry describes quick and irreversible one pot conjugation reactions that have a high reaction specificity, high yield of the desired product with only minimal byproducts.
Azides react with Alkyne-containing molecules in a Cu-catalyzed Alkyne-Azide cycloaddition, or with DBCO- (Dibenzylcyclooctine-) modified molecules in a Strain-promoted (Cu-free) Alkyne-Azide cycloaddition.
PolyAn equips glass slides, coverslips, polymer slides and 96-well plates with reactive surfaces. Please do not hesitate to contact us, if you would like to functionalize a different format or substrate with our 2D-Azide surface.
Selected Publications
Azide surface for the immobilization of Oligonucleotides (DNA/RNA):
- Choi, H. et al., `Light-guided molecular patterning for programmable multiplexed single-molecule manipulation´, bioRxiv, 2025, DOI: 10.1101/2025.04.30.651527.
- Verardo, D. et al., `Multiplex enzymatic synthesis of DNA with single-base resolution´, Sci. Advances, 2023, 9, 263. DOI: 10.1126/sciadv.adi0263.
- Valle-Orero, J. et al., `Strand switching mechanism of Pif1 helicase induced by its collision with a G-quadruplex embedded in dsDNA´, Nucleic Acids Research, 2022, 50, 8767. DOI: 10.1093/nar/gkac667.
- Rieu, M. et al., `Single-molecule kinetic locking allows fluorescence-free quantification of protein/nucleicacid binding´, Commun. Biology, 2021, 4, 1083. DOI: 10.1038/s42003-021-02606-z.
- Rieu, M. et al., `Parallel, linear, and subnanometric 3D tracking of microparticles with Stereo Darkfield Interferometry´, Sci. Advances, 2021, 7, 3902. DOI: 10.1126/sciadv.abe3902.
- Wang, Z. et al., `Detection of genetic variation and base modifications at base-pair resolution on both DNA and RNA´, Commun. Biology, 2021, 4, 128. DOI: 10.1038/s42003-021-01648-7.
Azide surface for the immobilization of Peptides:
- Howard, C.J, et al., `Solid-Phase Peptide Capture and Release for Bulk and Single-Molecule Proteomics´, ACS Chem. Biol., 2020, 15, 1401. DOI: 10.1021/acschembio.0c00040.
