PolyAn Beads for Flow Cytometry
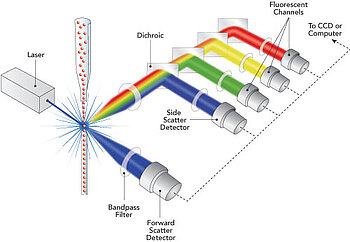
Flow cytometry is a laboratory technique used to analyze the physical and chemical characteristics of (biological) particles, such as cells or extracellular vesicles, by running them through a flow cell. As each bioparticle passes a beam of light, usually a laser, it scatters light and may emit fluorescence if tagged with specific dyes or antibodies. These signals are collected and measured to provide detailed information about particle size, complexity, and the presence of specific markers. Flow cytometry is widely used in immunology, cancer research, and clinical diagnostics to rapidly analyze thousands of bioparticles individually.
PolyAn offers beads both for flow cytometry calibration and for multiplexed bead assays.
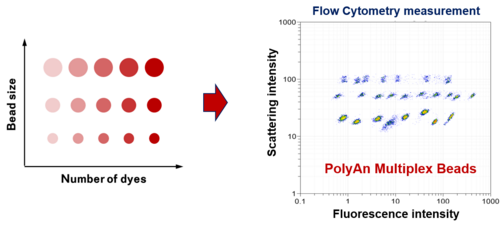
Multiplex Bead Assays for Flow Cytometry
PolyAn’s Multiplex Beads are designed for the development of multiplex assays that can be analyzed using a wide range of flow cytometers (open platform).
- PolyAn Red4 Multiplex Beads - up to 30 populations of single color-encoded beads
- PolyAn Blue Multiplex Beads - up to 10 populations of single color-encoded beads
- Combination of PolyAn Red4 30-plex and Blue 10-plex for up to 40 analytes
- Fluorescence Lifetime Beads for lifetime-multiplexed flow cytometry
PolyAn’s multiplex assay technology utilizes multiple bead populations differentiated by size and different levels of fluorescence intensity and/or fluorophores. PolyAn also offers customized development of multiplex sets for OEM-applications.
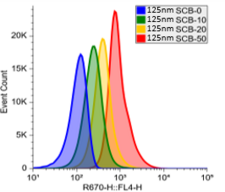
Calibration Beads for Flow Cytometry
PolyAn’s offers bead-based calibration tools for flow cytometry, that allow researchers to validate flow cytometer performance and standardize their results:
- Spectrum Calibration Microbeads - multicolor microbeads with different fluorescence intensities (up to 8 populations) for standard flow cytometers
- Spectrum Calibration Nanobeads - multicolor nanobeads with different fluorescence intensities (up to 4 populations) for nano flow cytometers
- Counting Beads with known particle number concentration
- Fluorescence Lifetime Beads for lifetime-based flow cytometry
Fluorescent beads of known intensity and size are commonly used in flow cytometry as calibration tools and controls, allowing researchers to standardize and validate the performance of the flow cytometer. They help ensure accurate alignment of the laser, proper detector sensitivity, and consistent fluorescence measurements across experiments. Fluorescent beads can also be used to create standard curves e.g. for quantifying fluorescence intensity or particle size, to simulate cells in assay development and training.

Selected Publications
PolyAn Red4 Multiplex Bead Assays for Flow Cytometry:
- Held, S. et al., `Physiological shedding and C-terminal proteolytic processing of TMEM106B´, Cell Reports, 2025, 44, 115107. DOI: 10.1016/j.celrep.2024.115107.
- Joseph, E. et al., `Design, Synthesis and Preclinical Evaluation of a brain-permeable PET Tracer for P2Y12R Imaging in the Brain´, ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-5461s.
- Laposchan, S. et al., `Development of Motif-Specific Monoclonal Antibodies for Global Protein Citrullination Detection with Minimal Cross-Reactivity to Homocitrullination´, bioRxiv, 2025, DOI: 10.1101/2025.03.27.645732.
- Pfisterer, M. et al., `The Aurora B-controlled PP1/RepoMan complex determines the spatial and temporal distribution of mitotic H2B S6 phosphorylation´, Open Biology, 2024, 14, 230460. DOI: 10.1098/rsob.230460.
- Papadogianni, G. et al., `Establishment and Functional Characterization of Murine Monoclonal Antibodies […]´, Antibodies, 2023, 12, 28. DOI: 10.3390/antib12020028.
- Riemenschneider, H. et al., `Targeting the glycine‑rich domain of TDP‑43 with antibodies prevents its aggregation in vitro and reduces neurofilament levels in vivo´, Acta Neuropath. Commun., 2023, 11, 112. DOI: 10.1186/s40478-023-01592-z.
- Rudan Njavro, J.et al., `Beneficial Effect of ACI-24 Vaccination on A-beta Plaque Pathology and Microglial Phenotypes in an Amyloidosis Mouse Model´, Cells, 2023, 12, 79. DOI: 10.3390/cells12010079.
- Kutzner, K. et al., `Phosphorylation of serine-893 in CARD11 suppresses the formation and activity of the CARD11-BCL10-MALT1 complex in T and B cells´, Sci. Signal., 2022, 15, 3083. DOI: 10.1126/scisignal.abk3083.
- Mishima, E. et al., `A non-canonical vitamin K cycle is a potent ferroptosis suppressor´, Nature, 2022, 608, 778. DOI: 10.1038/s41586-022-05022-3.
- Xin, S. et al., `MS4A15 drives ferroptosis resistance through calcium-restricted lipid remodeling´, Cell Death Differ., 2022, 29, 670. DOI: 10.1038/s41418-021-00883-z.
- Egia-Mendikute, L. et al., `Sensitive detection of SARS-CoV-2 seroconversion by flow cytometry reveals the presence of nucleoprotein-reactive antibodies in unexposed individuals´, Commun. Biol., 2021, 4, 485. DOI: 10.1038/s42003-021-02011-6.
PolyAn Beads for Flow Cytometry-based Assays:
- Anyanwu, N.C.J. et al., `Rigorous Process for Isolation of Gut-Derived Extracellular Vesicles (EVs) and the Effect on Latent HIV´, Cells, 2025, 14, 568. DOI: 10.3390/cells14080568.
- Choi, H.-K. et al., `Mechanotransduction governs CD40 function and underlies X-linked Hyper IgM syndrome´, bioRxiv, 2023, DOI: 10.1101/2023.07.23.550231.
- Deakin, C.T. et al., `Favorable antibody responses to human coronaviruses in children and adolescents with autoimmune rheumatic diseases´, Med, 2021, 2, 1093. DOI: 10.1016/j.medj.2021.08.001.
- Schauer, O. et al., `Motility and chemotaxis of bacteria-driven microswimmers fabricated using antigen 43-mediated biotin display´, Sci. Rep., 2018, 8, 9801. DOI: 10.1038/s41598-018-28102-9.
- Wooster, A.L. et al., `Expression and characterization of soluble epitope-defined major histocompatibility complex (MHC) from stable eukaryotic cell lines´, J. Immunol. Methods, 2018, 464, 22. DOI: 10.1016/j.jim.2018.10.006.
- Braun, A.C. et al., `Matrix Metalloproteinase Responsive Delivery of Myostatin Inhibitors´, Pharm. Res., 2017, 34, 58. DOI: 10.1007/s11095-016-2038-6.
PolyAn Beads for Imaging Flow Cytometry:
- Kanno, H. et al., `High-throughput fluorescence lifetime imaging flow cytometry´, Nat. Commun., 2024, 15, 7376. DOI: 10.1038/s41467-024-51125-y.
- Gouda; M. et al., `Surrogate gradient learning in spiking networks trained on event-based cytometry dataset´, Opt. Express, 2024, 32, 16260. DOI: 10.1364/OE.518323.
- Abreu, S. et al., `Flow cytometry with event-based vision and spiking neuromorphic hardware´, IEEE Xplore, 2023, 4139. DOI: 10.1109/CVPRW59228.2023.00435.
- Gouda; M. et al., `Improving the Classification Accuracy in Label-Free Flow Cytometry Using Event-Based Vision and Simple Logistic Regression´, IEEE Xplore, 2023, 7600608. DOI: 10.1109/JSTQE.2023.3244040.
PolyAn Beads for Calibration of Flow Cytometers:
- Rosendahl, P. et al., `Real-time fluorescence and deformability cytometry´, Nat. Methods, 2018, 15, 355. DOI: 10.1038/nmeth.4639.
PolyAn Beads for Lifetime-based Flow Cytometry:
- Kanno, H. et al., `High-throughput fluorescence lifetime imaging flow cytometry´, Nat. Commun., 2024, 15, 7376. DOI: 10.1038/s41467-024-51125-y.
- Kage, D. et al., `Tempo-spectral multiplexing in flow cytometry with lifetime detection using QD-encoded polymer beads´, Sci. Rep., 2020, 10, 653. DOI: 10.1038/s41598-019-56938-2.
- Kage, D. et al., `Lifetime encoding in flow cytometry for bead‑based sensing of biomolecular interaction´, Sci. Rep., 2020, 10, 19477. DOI: 10.1038/s41598-020-76150-x.
- Kage, D. et al., `Luminescence lifetime encoding in time-domain flow cytometry´, Sci. Rep., 2018, 8, 16715. DOI: 10.1038/s41598-018-35137.
