Streptavidin and Neutravidin Surfaces
for non-covalent oriented coupling of Biotin modified biochemical species
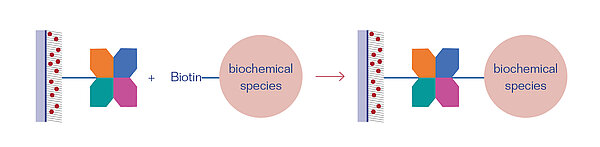
Streptavidin and Neutravidin are tetrameric proteins that can bind four Biotin molecules (vitamin B7) or any other Biotin-conjugated species with a very high specificity. The Streptavidin/Neutravidin-Biotin bond is one of the strongest, non-covalent bonds known in biochemistry, having a dissociation constant of KD = 10-15 mol/L. Thus, it is often applied in bioanalytical applications.
Streptavidin is a non-glycosylated protein purified from the bacterium Streptomyces avidinii. Neutravidin is a deglycosylated form of the native Avidin protein from egg white. Both Biotin-binding proteins can be distinguished by their isoelectric point, specificity, and non-specific binding:
| Avidin | Streptavidin | Neutravidin | |
|---|---|---|---|
| Molecular Weight | 67 kDa | 53 kDa | 60 kDa |
| Biotin Binding Sites | 4 | 4 | 4 |
| Isoelectric Point (pl) | 10 | 6.8-7.5 | 6.3 |
| Specificity | Low | High | Highest |
| Affinity for Biotin (KD) | 10-15 M | 10-15 M | 10-15 M |
| Nonspecific Binding | High | Low | Lowest |
Since PolyAn’s Streptavidin or Neutravidin matrices are covalently attached to the surface, the molecules are less susceptible to desorption in the presence of alkaline, acids, solutions of high ionic strength, or at high temperatures, compared to adsorptive immobilization.
PolyAn equips glass slides, coverslips and polymer slides as well as 96-well plates with Streptavidin and Neutravidin surfaces.
Please do not hesitate to contact us, if you would like to functionalize a different format or substrate with our Streptavidin or Neutravidin surfaces.
